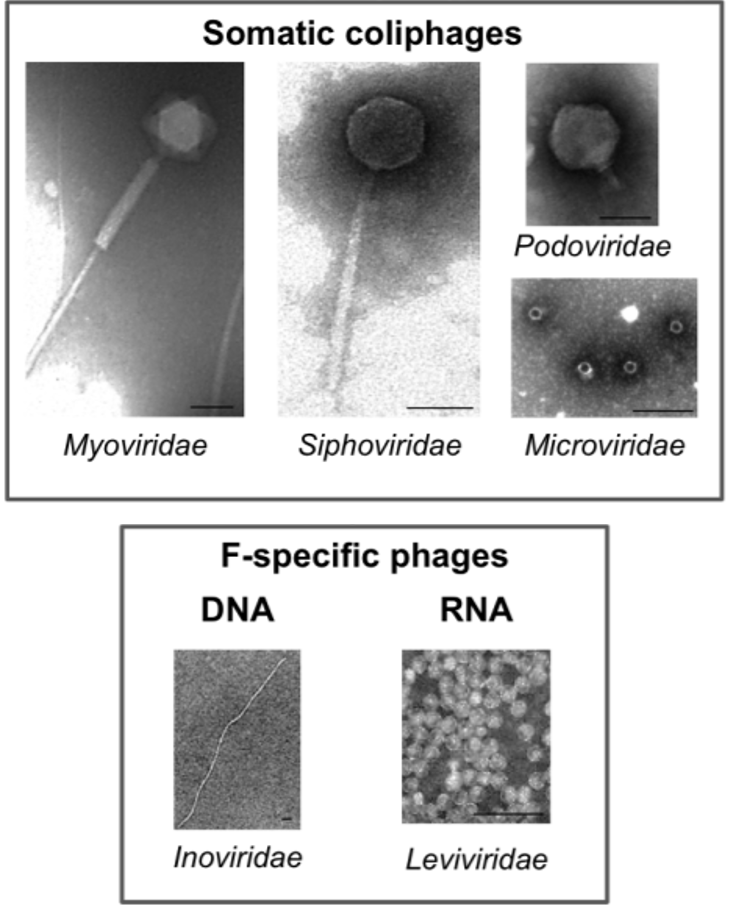
Coliphages are viruses able to infect coliform bacteria such as Escherichia coli or less frequently Shigella spp or Klebsiella spp.
E. coli is the most abundant bacteria in the lower intestine of humans and animals. Therefore, coliphages, which are non-pathogenic viruses, are the most common in the intestine.
Besides, it has been shown that coliphages do not replicate in the environment because of unfavourable conditions. Thus, coliphages found in the environment mainly come from faecal contaminations and can be used as an indicator of water microbiological quality.
Main characteristics of coliphages
In water, we mainly focus on two kinds of coliphages: somatic coliphages and F-specific RNA bacteriophages.
| Somatic coliphages | F-specific RNA bacteriophages | |
| Infection method | Through the cell wall | Through fertility (F) pili |
| Size | Variable (≈ 50-120 nm) | 21-30 nm |
| Genome | Single or double-stranded DNA | Single-stranded RNA |
| Most known family | Myoviridae, Podoviridae or Microviridae | Leviviridae |
| Most used surrogate | ϕX174 | MS2 |
The term « total coliphages », that we can find in some regulations, means both somatic coliphages and F-specific RNA bacteriophages.
What is the best indicator of faecal contamination / treatment efficiency?
Are somatic coliphages a better indicator of faecal contamination than F-specific RNA bacteriophages?
It is a matter of debate. Scientific studies appear to show that somatic coliphages are generally more abundant in water than F-specific RNA bacteriophages. However, the opposite seems to be true in ground water or in recycled water treated with UV light. Besides, from a purely technical point of view, enumeration of somatic coliphages is much easier.
What is certain is that in comparison with bacterial indicators, coliphages are less sensitive to disinfection processes and survive longer in the environment. Furthermore, viruses migrate farther and faster in soils than bacteria. Thus, water can be contaminated with human enteric viruses, even in the absence of traditional bacterial indicators (coliforms, E. coli). The report from French Agency for Food, Environmental and Occupational Health & Safety (ANSES, n° 2018-SA-0027), published in 2018, points out that bacteriophages are excellent indicators of treatment efficacy against viruses.
What are the regulations?
For some years now, many regulations around the world have introduced the analysis of coliphages to monitor the water quality (e.g. Australia, Europe, some states of the USA…). These new microbiological criteria concern the water intended for human consumption as well as treated wastewater. The existing regulations recommends analysing either somatic coliphages or F-specific RNA bacteriophages, or both.
In Europe, the enumeration of somatic coliphages is introduced in the new EU Drinking Water directive 2020/2184. They must be checked for in raw water. If the result is above 50 PFU per 100 ml, then, the water after treatment must be analysed.
Furthermore, this new directive introduces Water Safety Plans (WSPs). These management plans require the implementation of a new global strategy of risk prevention. Therefore, managers must have new indicators, such as somatic coliphages. This revision was published on December 23th, 2020.
Enumeration of coliphages is also required by the EU regulation on minimum requirements for water reuse published in June 2020. In this case, total coliphages should be analysed in the intake and output water of the treatment plant. A 6-log reduction is required for water intended for agricultural irrigation.
How to detect coliphages?
To meet these new regulatory requirements, laboratories must implement suitable analytical methods. Based on the ANSES report, in 2018 in France, only one laboratory was accredited for coliphage analysis, and only for F-specific RNA bacteriophages.
EN ISO 10705-1 and 10705-2 set out the method to enumerate respectively F-specific RNA bacteriophages and somatic coliphages using double agar layer.
However, these Standards only offer to inoculate 5 ml of water on 20 different petri dishes to analyse 100 ml samples. This method is extremely time and effort consuming, as well as costly. Therefore, it cannot be used as a routine method.
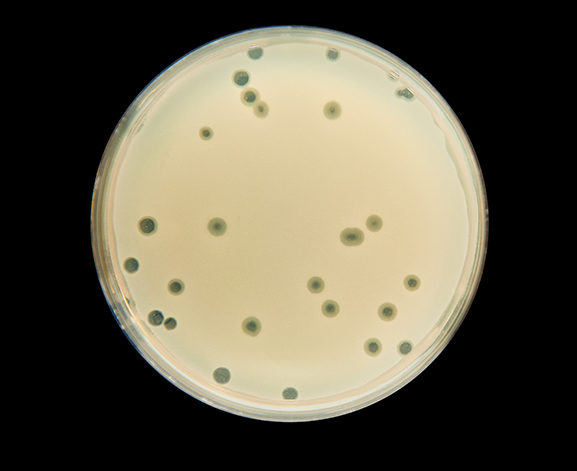
However, part 3 of this standard recommends a few solutions. Based on several studies, concentration on membrane filter is the easiest and cheapest to implement. It is particularly well adapted to observe the 4 to 6 LOG reductions required for the analysis of low turbidity water such as drinking water or reuse water.
This is why GL BIOCONTROL offers the concentration kit VIRAPREP® already used by several analysis laboratories.